Lesson 2
How the water pressure and air volume is affected during diving
By the end of this section, I should be able to answer these questions:
1. What is the relationship between my depth in water and the pressure?
2. What is the pressure change for each 10 metres/33 feet of depth change?
3. What is the relationship between pressure, and the volume and density of air?
4. If I take a volume of air from one depth to another depth, how much will the volume and density change?
Depth and Pressure
Right now, you are under pressure exerted by the air in the atmosphere that surrounds you. It’s actually the weight of the air. At sea level the pressure is fairly uniform, and expressed as one bar (metric) or one atmosphere (imperial – abbreviated ata). Underwater, you’re under more pressure because water also has weight, which combines with the atmosphere’s weight (pressure). Because water is much denser and heavier than air, 10 metres/33 feet exerts the same pressure as the whole atmosphere. Therefore, the pressure increases by one bar/ata for each 10 metres/33 feet you descend (go down). Likewise, it decreases one bar/atmosphere for every 10 metres/33 feet you ascend (come up). So:.

- At 0 metres/feet (sea level), the total pressure is 1 bar/ata.
- At 10 metres/33 feet, the total pressure is 2 bar/ata – one of air plus one of water.
- At 20 metres/66 feet, the total pressure is 3 bar/ata.
- At 30 metres/99 feet, the total pressure is 4 bar/ata.
Pressure and Air Volume and Density
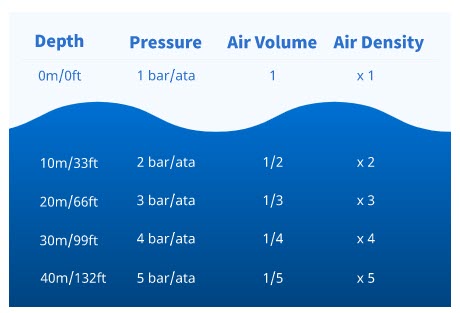
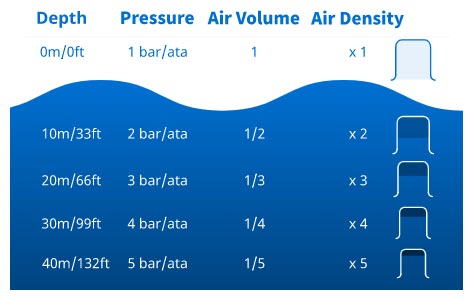
Water can’t be compressed so its volume and density don’t change with pressure changes. And, since your body tissues are mostly made of water, you don’t feel pressure changes on most of your body while diving. But, pressure changes do change the volume and density of air (or any other gas).
As the pressure increases – as you go deeper – a gas volume decreases because the gas molecules get compressed. The gas density increases because all the molecules are there, but they’re packed into a smaller area.
This is one of the most important principles you learn, because as a diver, it affects all the air spaces in, or in contact with, your body. These include your ears, sinuses, lungs, mask and, when using one, a dry suit. You’ll also learn that this principle affects controlling your buoyancy, how long your air supply lasts and some important safety rules.
Air volume and density change proportionately with pressure. This means if you go from the surface to 10 metres/33 feet, you double the pressure to 2 bar/ata (1 air plus 1 water), a given air volume halves, and its density doubles. If you go to 20 metres/66 feet, the pressure is 3 bar/ata (1 air plus 2 water). An air volume would be one-third the surface volume, and the density would triple.
Example:

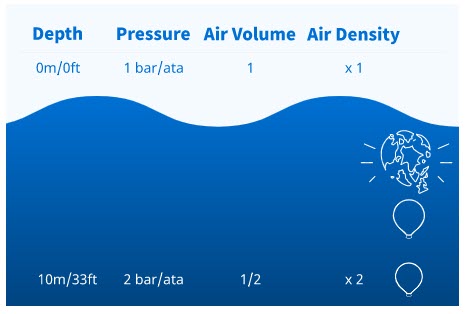
This predictable relationship between pressure and air (gas) volume and density exists with every depth change whether descending or ascending. For example, suppose you have 1 litre of air at 30 metres/99 feet. What would the volume and density be at the surface?
The pressure at 30 metres/99 feet is 4 bar/ata (3 of water plus 1 of air).
- The pressure at the surface is 1 bar/ata, so the pressure is 1/4th the pressure at 30 metres/99 feet.
- One litre of air brought to the surface from 30 metres/99 feet will expand to 4 litres at the surface. The air density would be 1/4th the density at 30 metres/ 99 feet.
Now suppose you fill a balloon completely and seal it at 10 metres/33 feet.
What happens as you ascend? The balloon expands, growing larger until it stretches past its failure point and bursts. To prevent this, you’d leave the balloon unsealed and vent some of the expanding air as you ascend.
Lesson Quiz
Module One

I'll take you diving!
Copyright © Larry Wedgewood Scuba Instruction All Rights Reserved

